Daiichi Sankyo enrols first patient in Phase III acute myeloid leukaemia trial
17 October 2016 | By Niamh Louise Marriott, Digital Content Producer
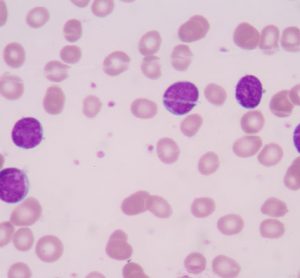
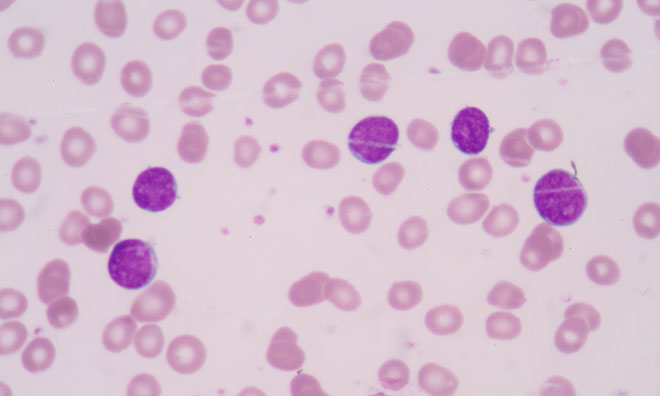
Daiichi Sankyo has enrolled their first patient in the global phase 3 QuANTUM-First study evaluating the oral FLT3-ITD inhibitor quizartinib in patients...