UCB and Amgen’s Osteoporosis study shows bone mineral density gains
18 November 2016 | By Niamh Louise Marriott, Digital Content Producer
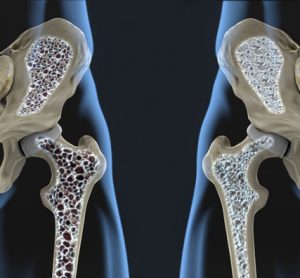
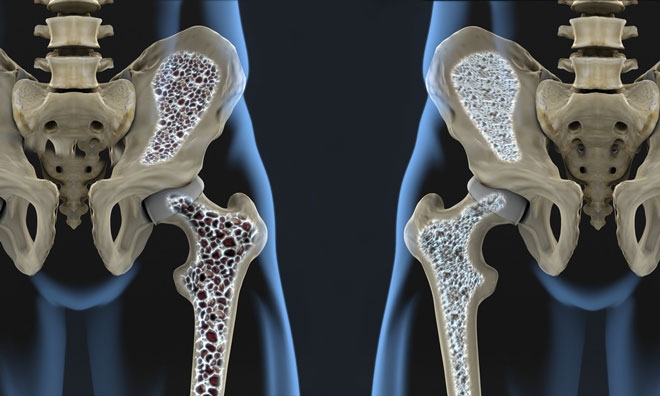
The BRIDGE study involved 245 men with osteoporosis randomised 2:1 to receive either 210 mg romosozumab or placebo subcutaneously once monthly for 1 year...