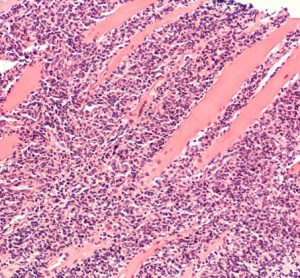
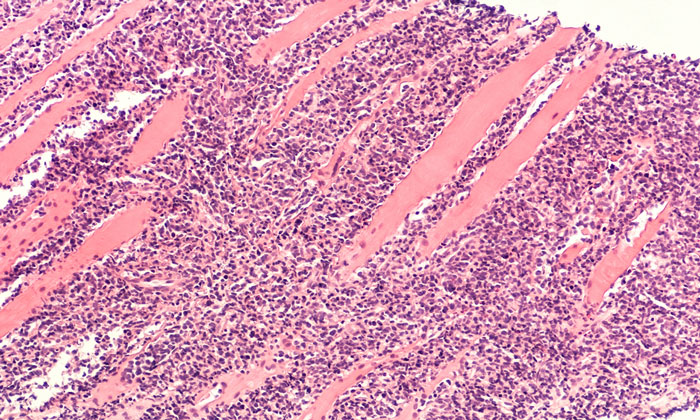
Bioprocessing & Bioproduction In-Depth Focus 2021
In this in-depth focus, discover how biological and technological advances could enhance the clinical capabilities of CAR T-cell therapies and explore why clinicians need to be better educated about the development and licencing of biosimilars.











![Male Thai child receiving oral polio vaccine from healthcare worker [Credit: frank60 / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Oral-vaccine-300x278.jpg)
![Male Thai child receiving oral polio vaccine from healthcare worker [Credit: frank60 / Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/Oral-vaccine.jpg)