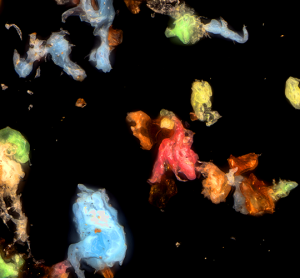
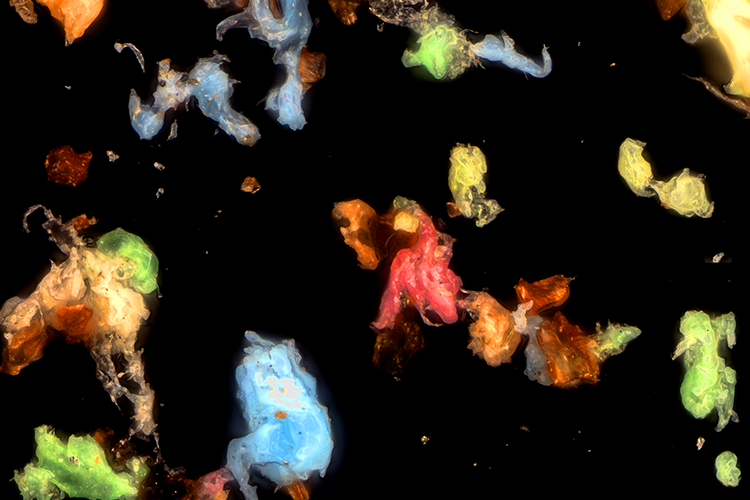
Analytical method transfer and the supplier change processes for bacterial endotoxin testing
Changes to lab setups become necessary for many reasons, but the important role of quality control should remain consistently accurate. Tim Sandle and Kerry Skinner describe the details to consider when transferring an analytical method between laboratories or when changing your reagent supplier.