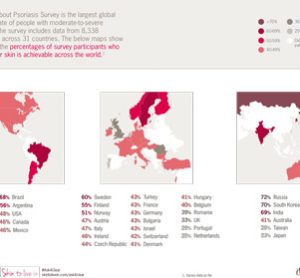
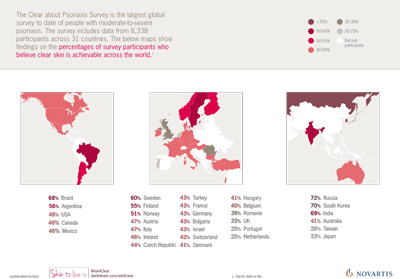
Novartis wins “Discovery of the Decade” and “Best Biotechnology Product” awards
31 October 2016 | By Niamh Louise Marriott, Digital Content Producer
Novartis has been awarded the 2016 Prix Galien USA Award "Discovery of the Decade" Award for Best Pharmaceutical Product for the drug Gleevec, as well as...