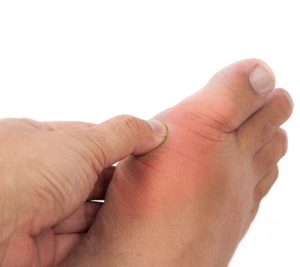
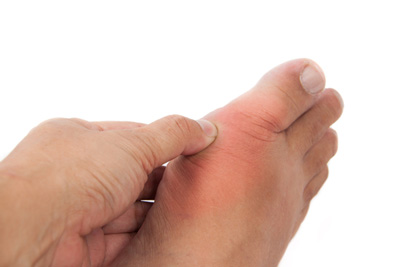
AstraZeneca’s Zurampic approved in the EU for patients with gout
19 February 2016 | By Victoria White
In combination with the current standard of care, Zurampic provides a dual mechanism of action to increase excretion and decrease production of uric acid...