United efforts to accelerate drug development
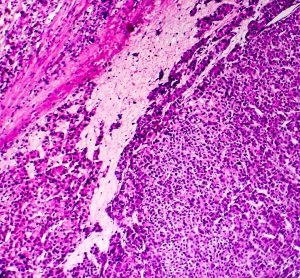
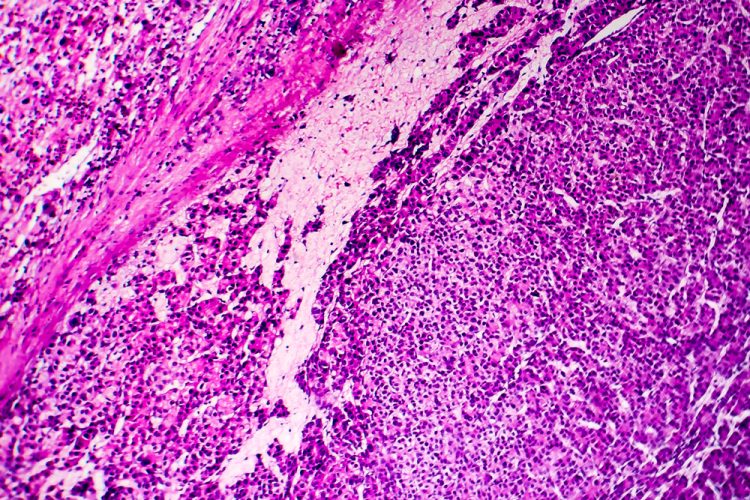
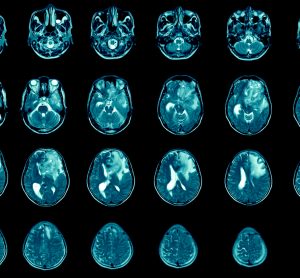
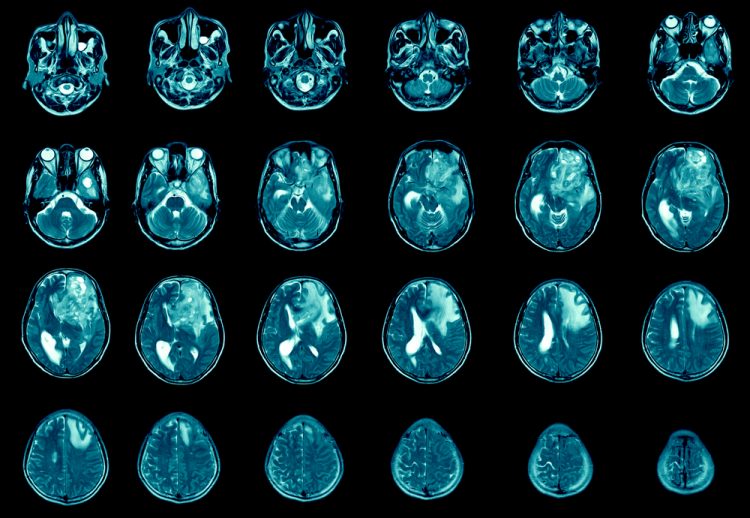
From the Cancer Drug Development Forum (CDDF), John Smyth (Chairman), Axel Glasmacher (Treasurer) and Jaap Verweij (Managing Director) clarify the importance of a collaborative industry approach to cancer therapy development and highlight the most pressing challenges for discussion in their meetings and workshops.