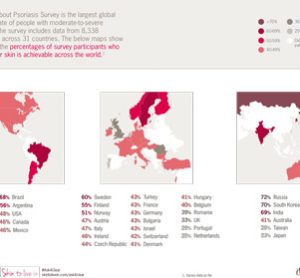
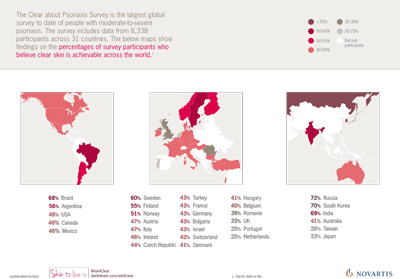
NICE recommends Novartis’ ankylosing spondylitis drug
29 September 2016 | By Niamh Louise Marriott, Digital Content Producer
NICE are recommending it as an option to treat adults with active ankylosing spondylitis (AS) who have responded inadequately to conventional therapy...