Actualising the power of antibody-drug conjugates as cancer therapeutics
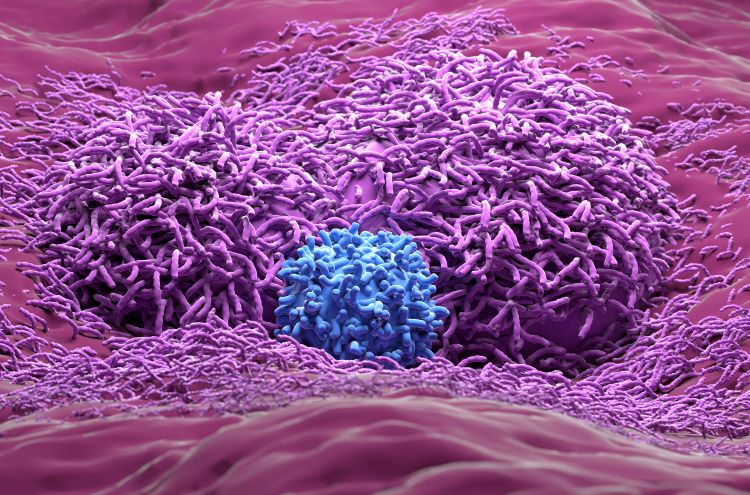
Antibody-drug conjugates (ADCs) are designed to be highly-targeted therapies with the potential to maximise the potency of a treatment while reducing unwanted side effects on healthy tissues. Some 20 years after the first ADC product approval, Dominik Schumacher and Jonas Helma-Smets of Tubulis GmbH discuss how improved and optimised technologies…


















![Close up of Amgen sign at its headquarters in Thousand Oaks, California, USA [Credit: JHVEPhoto/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/amgen-2-e1660136652889-300x278.jpg)
![Close up of Amgen sign at its headquarters in Thousand Oaks, California, USA [Credit: JHVEPhoto/Shutterstock.com].](https://www.europeanpharmaceuticalreview.com/wp-content/uploads/amgen-2-e1660136652889.jpg)